Nature of C - X Bond in Haloalkanes
Nature of C - X Bond in Haloalkanes: Overview
This topic covers concepts, such as, Polarity of Carbon-Halogen Bond in Haloalkanes, Bond Length of Carbon-Halogen Bond in Haloalkanes, Bond Enthalpy of Carbon-Halogen Bond in Haloalkanes & Dipole Moment of Carbon-Halogen Bond in Haloalkanes etc.
Important Questions on Nature of C - X Bond in Haloalkanes
The most polar compound among the following is:
is a gas, is a liquid, and are solids at room temperature. The highest bond enthalpy is observed in ______
The decreasing order of bond enthalpies of the following alkyl halides is:
(i)
(ii)
(iii)
(iv)
The lowest stability of carbocation is in the compounds
Explain the nature of bond in:
Explain following-
Directional nature of halogen atom in haloarenes.
From which of the following sequences does the reactivity of alkyl halides in the nucleophilic substitution reaction follows:
Which of the following are arranged in the decreasing order of dipole moment?
Arrange the following alkyl halides in order of dehydrohalogenation;
Which of the following statements is true is case of alkyl halides?
Of the following compounds which will have a zero dipole moment ?
The correct sequence of dipole moments among the chlorides of methane is-
Which two of the following molecule has dipole moment?
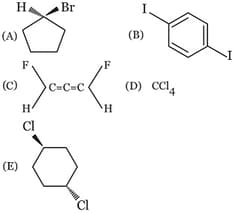
Select the correct statement.
Discuss the relative dipole moments of and .
